17th International Conference on Cytopathology and Histopathology
Vancouver, Canada

Aleksandra Zuraw
Charles River Laboratories Montreal, Canada
Title: Role of digital pathology in drug development process
Biography
Biography: Aleksandra Zuraw
Abstract
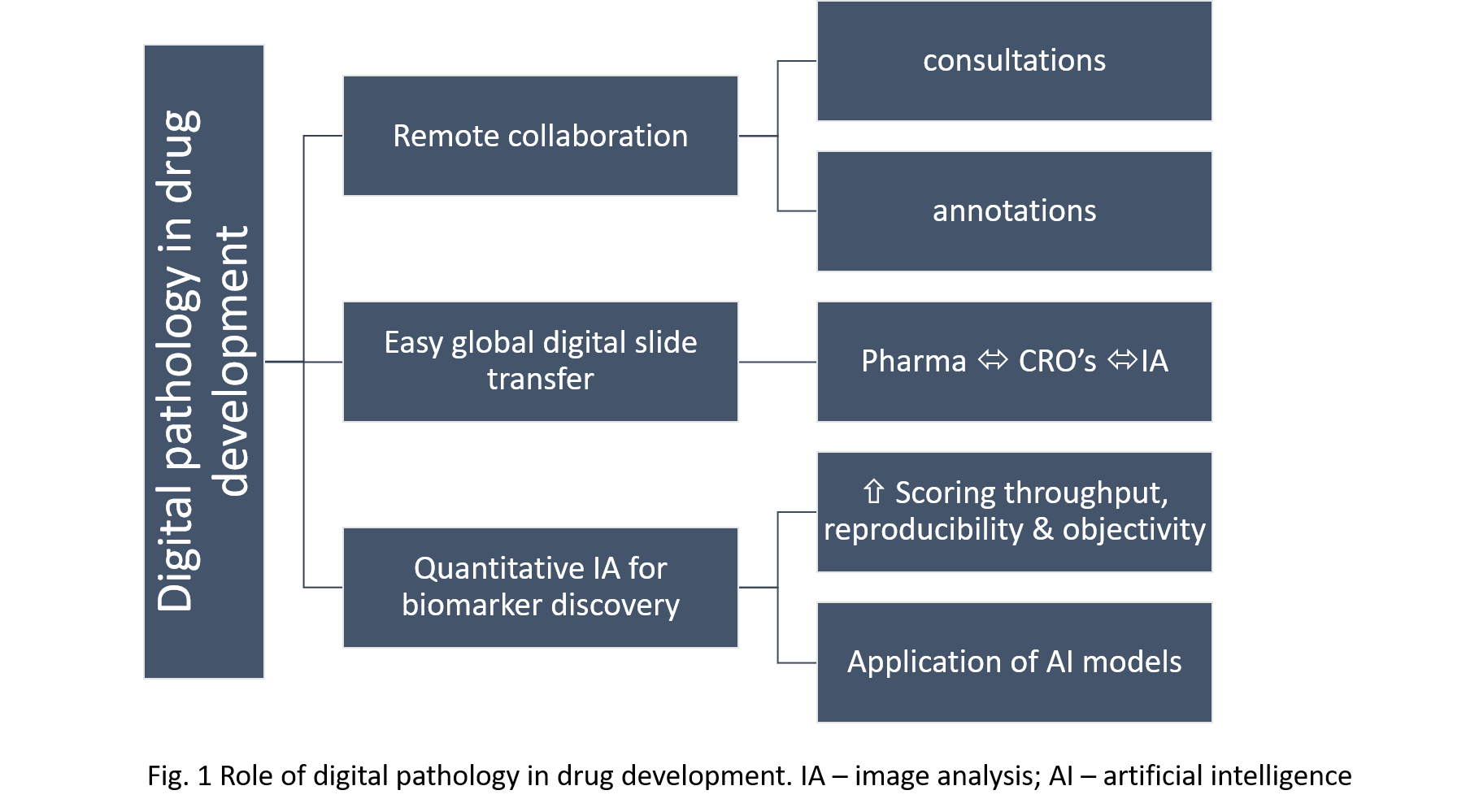
Digital pathology is the process of performing pathology services using computers and digital images instead of microscopes and glass slides. With the increasing capabilities of pharmaceutical companies and contract research organizations to rapidly perform whole slide imaging and convert glass slides into digital images, there is a great potential to unlock the benefits of this technology for the drug development process. Pathologists are crucial members of drug development teams and are engaged in every step of the process, contributing significantly to the discovery, preclinical and clinical phases. Implementation of digital pathology workflows within and across organizations empowers them and benefits the drug development process in multiple ways. Often pathologists involved in pharmacological studies are geographically dispersed. The use of digital pathology allows them to communicate fast and perform slide consultation in real time regardless of location, which increases the efficiency of their work. Multiple pathologists can view, annotate and comment on the same slide simultaneously. The digitization of slides enables image analysis-powered quantitative measurements of biomarkers, lesions and abnormalities, which increases the throughput, reproducibility and objectivity of pathologists’ scoring. As tissue research is cross-disciplinary, access to digital pathology for different groups involved in drug development, especially the DVM and MD pathologists, helps them work more collaboratively and better understand their contributions. This technology is still considered novel, and in many institutions, its implementation is met with skepticism. Nevertheless, making it accessible and user-friendly for pathologists, standardizing it and applying it broadly across organizations, will undoubtedly accelerate and advance drug development.